
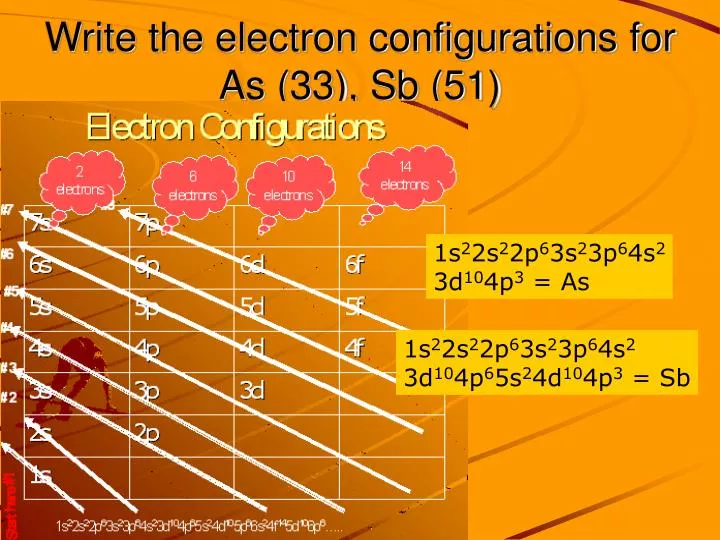
This element is also one of the heavier pnicogens but is very brittle and bad at conducting electricity. Antimony can either lose the three p electrons, resulting in a 3+ charge, or lose all five electrons to result in a 5+ charge.
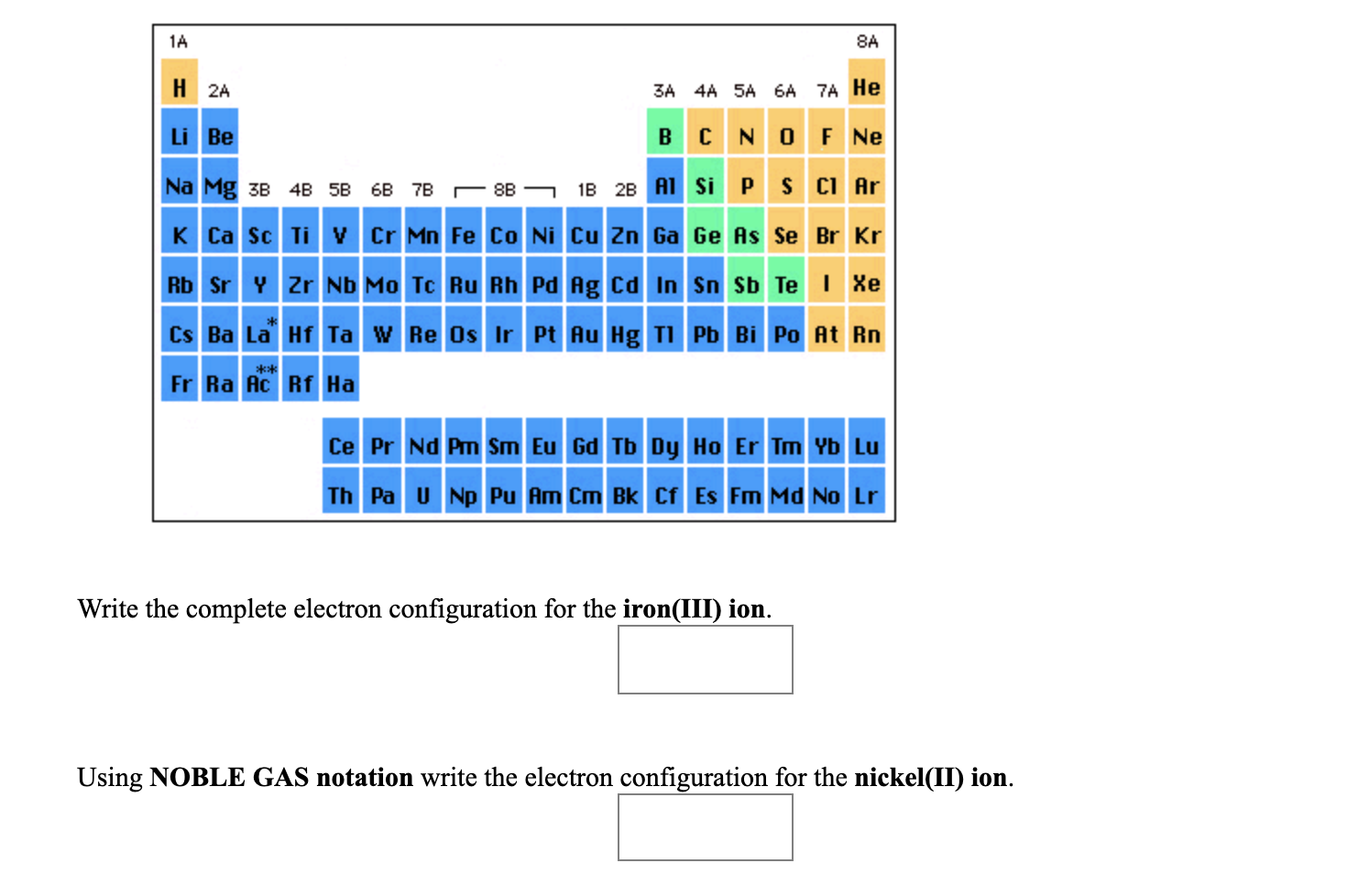
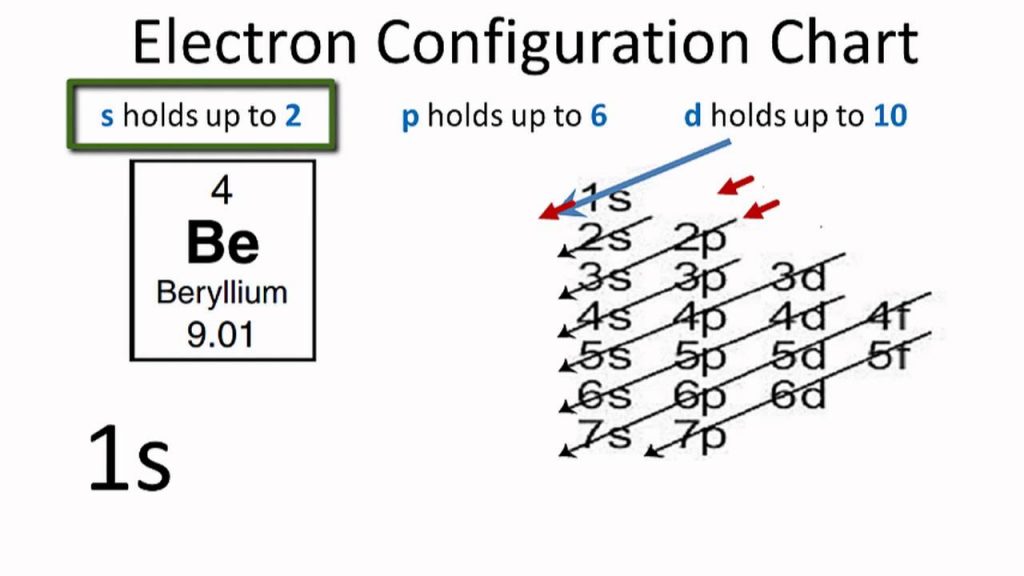
Furthermore, all pnictogens have five valence electrons two of these electrons are paired and exist in the s subshell, while the remaining three electrons exist in the p shell, unpaired. Pnictogens are special because they form strong double and triple covalent bonds (learn more about covalent bonds here) to produce stable compounds.Īntimony can react with almost all of the metals on the periodic table to form pnictides. Along with antimony, other elements of this group include nitrogen (learn about the discovery of nitrogen), phosphorus, arsenic, bismuth, and ununpentium. Pnictogen elements are members of the nitrogen group of the periodic table. Antimony has an electron configuration of 4d 105s 25p 3. It lies below arsenic, and above bismuth, and it has properties similar to both of those elements. It lies to the right of tin, and to the left of tellurium. One theory of Wolfgang Amadeus Mozart’s early death is that his doctor poisoned him with a toxic antimony medication.Īntimony, atomic symbol Sb, has atomic number 51 on the periodic table.Thankfully, people now know better to not follow these dangerous practices. However, it has been widely used for different medicinal purposes, including laxatives. Antimony is poisonous and should never be inhaled or ingested.There are biblical references of antimony in the Old Testament, where Queen Jezebel uses an antimony compound for makeup.This relates to how antimony was used to make black eye makeup. Stibium originates from the Greek word “stibi”, meaning mark. Antimony’s symbol is Sb, which comes from “stibium”.Its name’s origin comes from the Greek words, “anti” and “monos”, meaning “not alone” because it is always found with another element.Antimony can be thought of as a lonely element because it is never found alone It is always combined with another element.
Known since ancient times, this metal has widespread uses, including black eye makeup. This metal has a flaky texture and is hard and brittle. The element antimony is a shiny, silver-colored semi-metal of the nitrogen group of the periodic table.


 0 kommentar(er)
0 kommentar(er)
